Author: Ed Gover
NEW YORK – Swedish firm Affibody launched a new Phase II study of its HER2 PET imaging agent tezatabep matraxetan to identify metastatic breast cancer patients who may respond to treatment with Daiichi Sankyo and AstraZeneca's antibody-drug conjugate Enhertu (trastuzumab deruxtecan), the company announced Tuesday.
Please register for free here and read more
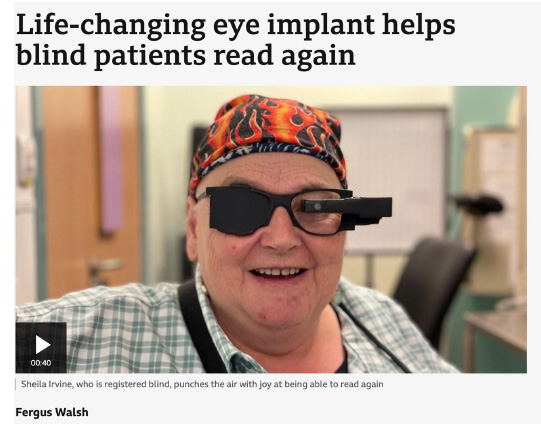
A group of blind patients can now read again after being fitted with a life-changing implant at the back of the eye.
A surgeon who inserted the microchips in five patients at Moorfields Eye Hospital in London says the results of the international trial are "astounding".
Sheila Irvine, 70, who is registered blind, told the BBC it was "out of this world" to be able to read and do crosswords again. "It's beautiful, wonderful. It gives me such pleasure."
Please cclick here to read the full story
The Nordic Life Science (NLS) Days took place this week, October 13–14, in Gothenburg, Sweden. Labiotech was on site to take the pulse of the Nordic biotech scene, and, more specifically, of Sweden’s growing role within it.
Within Sweden, Gothenburg isn’t usually the first place people think of when they picture biotech, yet a closer look reveals a city quietly becoming one of the country’s most dynamic life science hubs. You could even argue that much of what’s happening here simply doesn’t exist anywhere else.

Please click here to read more
With a legacy rooted in global scientific excellence, Sweden is quietly building one of Europe’s most dynamic biotech ecosystems - despite lacking a big pharma anchor and facing persistent funding gaps.

- CPHI Frankfurt 2025 will highlight advancements in pharmaceutical process control, patient safety, and efficiency, addressing automation, material performance, and regulatory compliance challenges.
- BENEO's galenIQ™ excipient offers robust granule formation and high-strength tablets for continuous manufacturing, enhancing patient compliance with its pleasant taste.

Please click here to readmore
Welcome to the BioPharm International® weekly news roundup for the week of Oct. 13, 2025. In this video feature, the editors highlight each week’s industry news in an easy-to-consume, fun format. New roundups will drop every Friday, so be sure to come back each week. And be sure to check the links below for more in-depth coverage of each story.
Biologics, obesity R&D partnerships, and China collaborations are driving pharmaceutical M&A and selective venture capital funding in the third quarter of 2025, according to a new report by J.P. Morgan.
Please click here to read more
This series, “The Situation We Faced,” is a CEO-type investigation interview into decision making, set within the context of other options, relative risk-reward situation, outcome and corporate culture ramifications, and market competitiveness positioning. So, sort of a war story reminiscence, but with clear technology or product context, some speculation about how other choices might have assisted or held things back.
As Hanns-Christian Mahler, CEO of Ten23 Health, explains the situation, “We saw up to 80% of the batch that had to be sorted out because of particulate defects in the final product, which eventually were clearly traceable to the primary packaging that was in use….
Please click here to read more
STOCKHOLM, SWEDEN – October 9, 2025. Karolinska Development AB (Nasdaq Stockholm: KDEV) today announces that its portfolio company PharmNovo has received approval from the Spanish regulatory authorities to initiate a phase 2a clinical trial of its drug candidate, PN6047, being developed as a treatment for neuropathic pain. The trial will be conducted in the EU, but has been fully aligned with the requirements defined by the U.S. Food and Drug Administration (FDA), earlier this year.
Please click here to read more

The first patient has been dosed in a Phase I clinical trial of breast cancer treatment ABY-271.
ABY-271 is a radiotherapeutic candidate being assessed as a potential treatment for HER2-positive metastatic breast cancer. The Phase I study evaluating the safety, tolerability and biodistribution of ABY-271 is a first-in-human open-label, two-stage, randomised trial being conducted in Sweden and Germany. The first patient was enrolled at Uppsala University Hospital in Sweden.
ABY-271 is an Affibody molecule that targets HER2-expressing tumours and is labelled with the radioisotope lutetium-177, which emits cytotoxic beta radiation, exerting irreversible damage to the tumour cells upon binding.